Technology
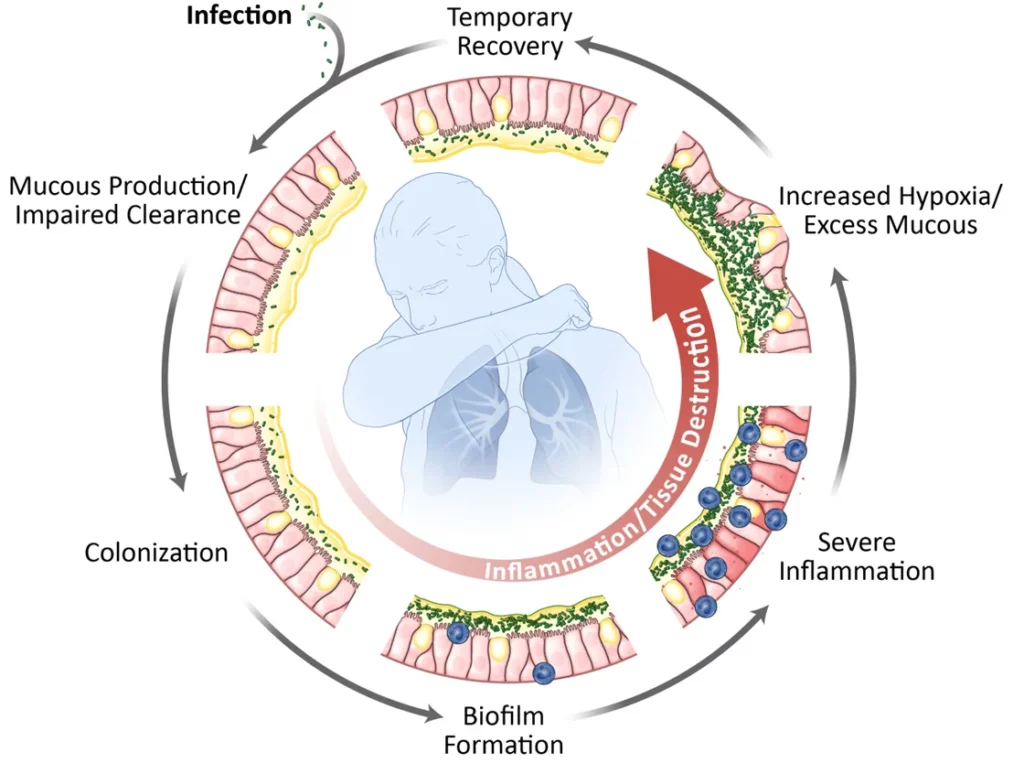
Vicious Cycle of Infection and Inflammation
The progression of many lung diseases that ultimately leads to loss of lung function is driven by upregulated inflammation, typically as a byproduct of environmental stressors or the damage that occurs when the lungs are colonized with certain microorganisms. Bacteria develop biofilms to protect themselves from antibiotics and immune cells. The persistent inflammation leads to excessive mucous production and gradual tissue destruction, which occurs again and again in the diseases we are targeting.
Nitric Oxide – Dual Mechanism of Action
Central to the Vast technology platform is the dual anti-inflammatory and antimicrobial activity of nitric oxide. Nitric oxide is a gas, produced by the body for many key functions of everyday life. Certain cells of the immune system or airway lining produce higher levels as part of the innate immune response. However, in some diseases, or in the presence of select pathogens, the nitric oxide biosynthesis pathways are overwhelmed or turned off.
Nitric oxide produced in higher concentrations has a critical anti-inflammatory effect by serving as a checkpoint inhibitor of the NLRP3 inflammasome. Decreasing production of IL-1B and recruitment of neutrophils, accelerating the rate of clearance in tissue macrophages, downstream reductions in IL-17, and decreased ICAM expression on endothelial cells limiting leukocyte recruitment are some of the other known anti-inflammatory mechanisms of nitric oxide which could be beneficial to patients.
As an antimicrobial, nitric oxide is a unique alternative to chronic antibiotics because it has broad-spectrum activity. Nitric oxide kills bacteria via multiple mechanisms including nitrosative and oxidative stresses that damage DNA, lipid membranes, proteins, and iron-sulfur clusters leading to an extremely low propensity for drug resistance.
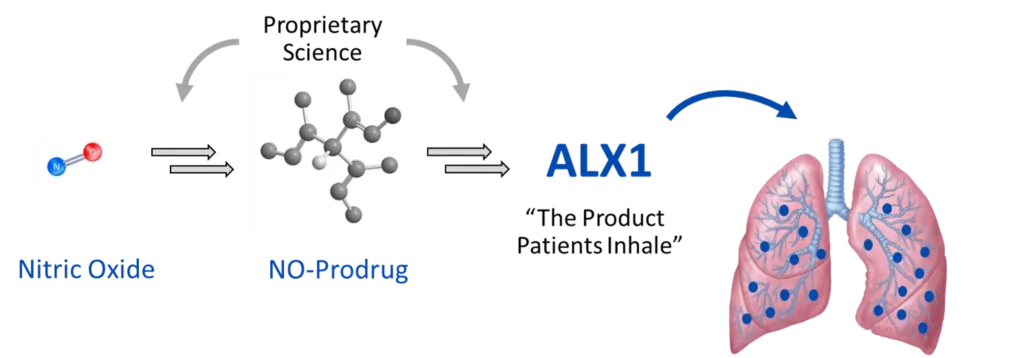
ALX1 Drug Candidate
Our drug candidates store nitric oxide in prodrug form, releasing controlled amounts for sustained durations. When combined with deep inhalation formulation science for delivering drug candidates to the lungs, the result is an aerosolized therapy targeting the specific needs of the patient population. ALX1 contains a water soluble, small molecule prodrug (<500 Da) that releases the pharmacologically active metabolite, Nitric Oxide (NO) with sustained release duration of up to 8 hours. This candidate is formulated as a buffered aqueous solution for oral inhalation via a customized PARI eFlow® nebulizer. The ALX1 pharmacology program includes in vitro and in vivo evidence of anti-inflammatory target engagement as well as antimicrobial efficacy against Pseudomonas aeruginosa and Non-tuberculous mycobacterium infections.
Pipeline
Vast is aiming to address chronic lung conditions where the underlying disease creates ‘at-risk’ patient populations susceptible to multi-drug resistant microbial infections and chronic inflammation.
Bronchiectasis
Lead Optimization
Preclinical
Phase 1
Phase 2
Phase 3
Cystic Fibrosis
Non-CF Bronchiectasis
Other Respiratory
Lead Optimization
Preclinical
Phase 1
Phase 2
Phase 3
NTM Disease
COPD
Other Nitric Oxide Prodrugs
Vast has a library of other nitric oxide prodrugs that can also leverage this dual mechanism of action in inhaled form. Additional formulations could include dry powder inhalers, meter dose inhalers, or fixed dose aerosolized combinations of two active agents to target a broad range of severe respiratory infections. Once deposited in the lungs, the prodrugs begin to degrade with total nitric oxide release durations ranging from 1 to 24 hours depending on the specific chemistry.
The company has a broad, worldwide patent portfolio including issued patents and pending applications at the composition of matter, methods of manufacture, formulation science, and adjuvant therapy levels of our strategy. The issued patents cover ALX1, nitric oxide-releasing compounds, and their pharmaceutical formulations.

